“I am very pleased with the results from the TMS therapy. I’ve suffered from depression for 20 years and doctors have tried many different antidepressant medication regimens. I have never seen the results I’ve seen with the TMS therapy. I cannot begin to tell you how refreshing it is to finally see the glass half-full. Thank You!” – TBK, St. John Mental Health Clinic of Houston, TMS patient
TMS stands for Transcranial Magnetic Stimulation. TMS is non-invasive, meaning that it does not involve surgery and does not require any anesthesia or sedation. Patients remain fully awake and alert during TMS therapy sessions.
TMS is non-systemic, meaning that it does not enter the bloodstream nor affect other areas of the body like medications can.
TMS refers to a medical treatment which is delivered by a device that generates changing magnetic fields. TMS Therapy system generates highly concentrated magnetic fields which are rapidly switched on and off. These magnetic fields do not directly affect the whole brain; they only reach about 2-3 centimeters into the brain.
The device is positioned over the prefrontal cortex – the area of the brain which is being targeted when treating depression. These magnetic fields can create electrical activity in the nerve cells. It is well accepted by researchers and clinicians that the magnetic stimulation affects not only the targeted area (e.g. the prefrontal cortex), but also other areas of the brain connected to the prefrontal cortex, including the cingulate, hypothalamus, and thalamus.
There are many devices which can be used by clinicians. In this office, we utilize several different devices, including the NeuroStar Advanced Therapy System, the Magstim Rapid2 Therapy System, and the MagVenture Express TMS System.
The most common side effects of TMS include dizziness, headache, and minor scalp pain.
There is a risk of seizure with the use of Transcranial Magnetic Stimulation. Patients who have had a seizure or who have a medical condition which could increase the risk of having a seizure should share that information with Dr. St. John at their initial consultation.
TMS treatment systems create a magnetic field that could cause metal objects near the stimulation area to move or heat up. Patients with non-removable magnetic-sensitive metals or metallic devices implanted in their head or in body parts during stimulation could experience adverse effects from the treatment and should not receive TMS therapy. Types of non-removable devices include cochlear implants, deep brain stimulators, aneurysm clips or coils, and stents.
 Current literature indicates that approximately 50% of all treated patients who meet criteria for TMS (i.e. are considered treatment-resistant) respond to the treatment by bettering their score on a standardized rating scale by 50% or more. Approximately one-third of treated patients are so-called remitters, bettering their scores so much that they are considered depression-free.
Current literature indicates that approximately 50% of all treated patients who meet criteria for TMS (i.e. are considered treatment-resistant) respond to the treatment by bettering their score on a standardized rating scale by 50% or more. Approximately one-third of treated patients are so-called remitters, bettering their scores so much that they are considered depression-free.
To put these statistics in perspective, it is important to note that the patient population undergoing treatment with TMS Therapy is often excluded from new antidepressant development research studies.
In addition, the STAR*D study demonstrated that patients are likely to experience a poorer response to each new medication tried following successive treatment failures. For example, after the first medication attempt, about 30% of patients achieve remission. By the time the patient has attempted 3 different medications without improvement, the likelihood of the 4th medication leading to remission falls below 10%.
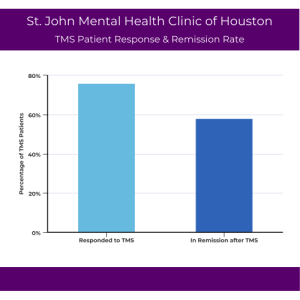
St. John Mental Health Clinic of Houston has used TMS in the fight against treatment-resistant depression since 2013. Since then, a response rate of 76% and a remission rate of 58% of the patient population was observed. This means that more than three-quarters of their patients cut their symptoms of depression in half, and almost two-thirds were symptom-free of their depression after completing a full course of TMS treatment in our Houston office.